
What is the Unique Device Identifier (UDI)
Description:
This guide defines the UDI, explains the role of the three FDA approved issuing agencies and outlines the key requirements manufacturers must follow.
Definition:
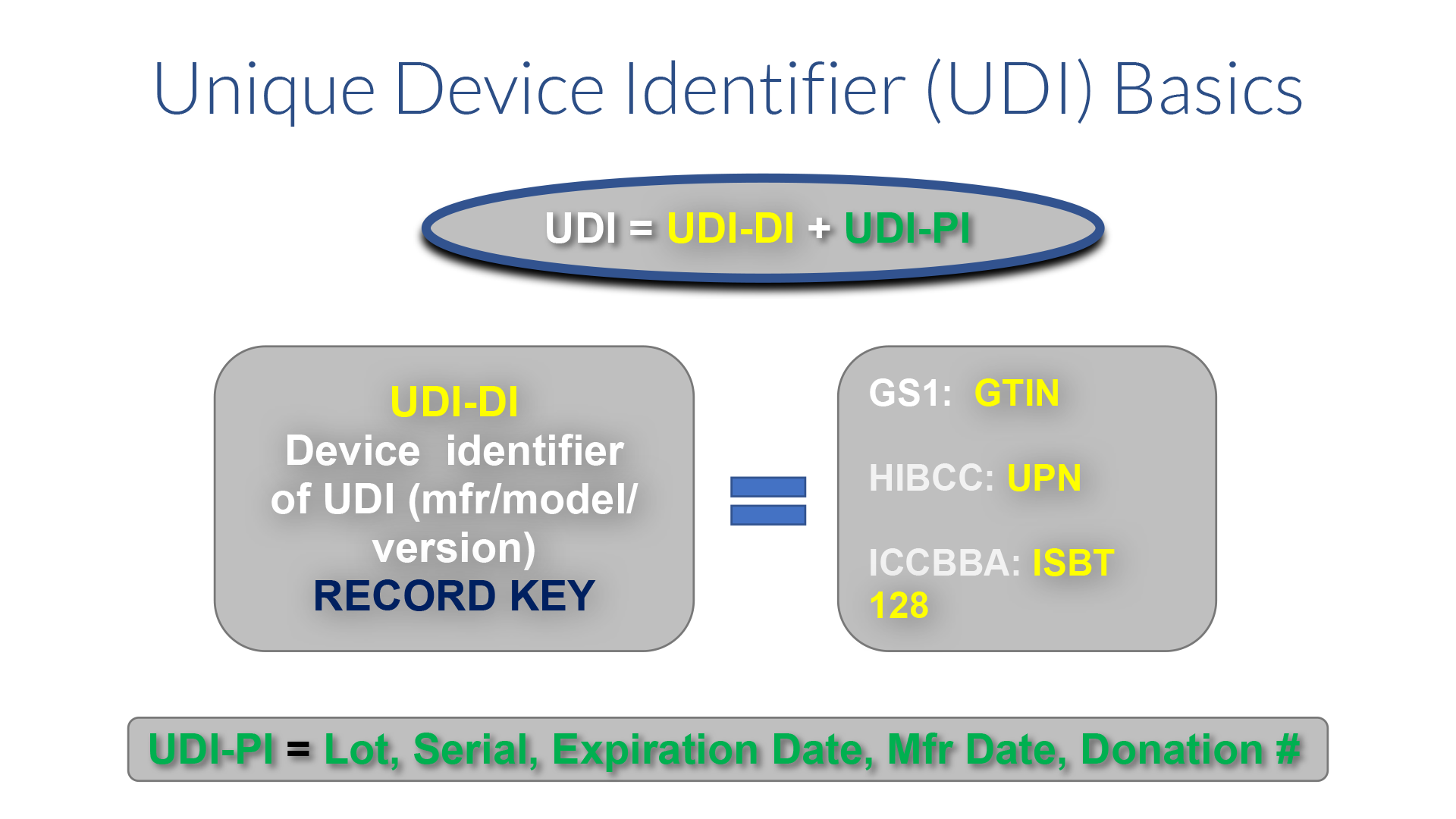
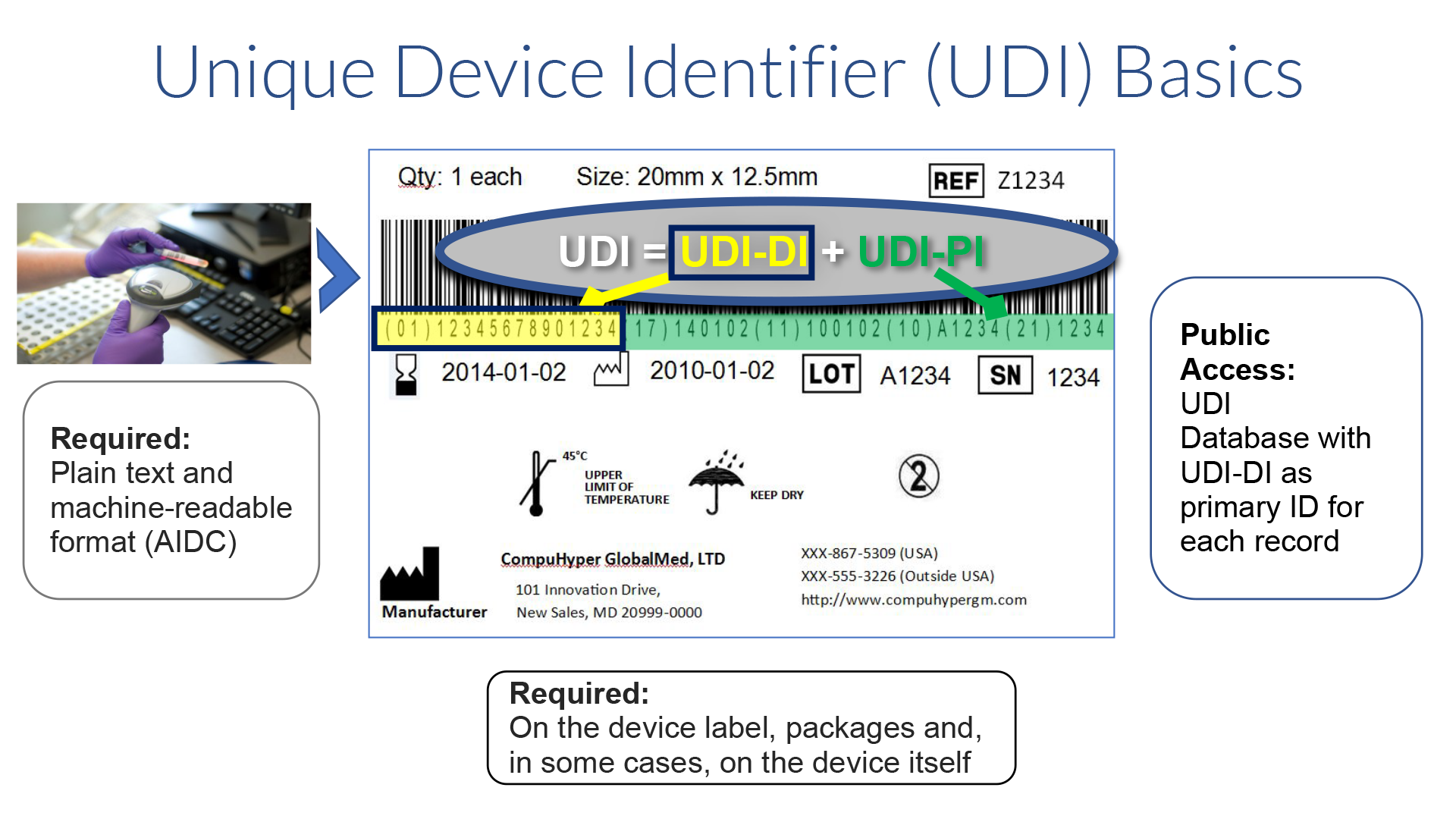
The FDA defines the UDI as a globally unique alpha or alphanumeric code that adequately identifies a medical device throughout its distribution and use. It is comprised of a mandatory, fixed Device Identifier (DI) which identifies the specific version or model and a variable Production Identifier (PI) which can identify one or more of the following – lot, batch, serial number, expiration date and manufacture date, and for human cell, tissue, cellular, or tissue based product (HCT/P) regulated as a medical device, the distinct identification code that allows the manufacturer to associate the HCT/P with the donor If the label of a device is not required to have a particular PI, then it is not required in the UDI. The UDI must appear on the label and package in both human readable and machine readable (AIDC) formats.
Issuing Agencies:
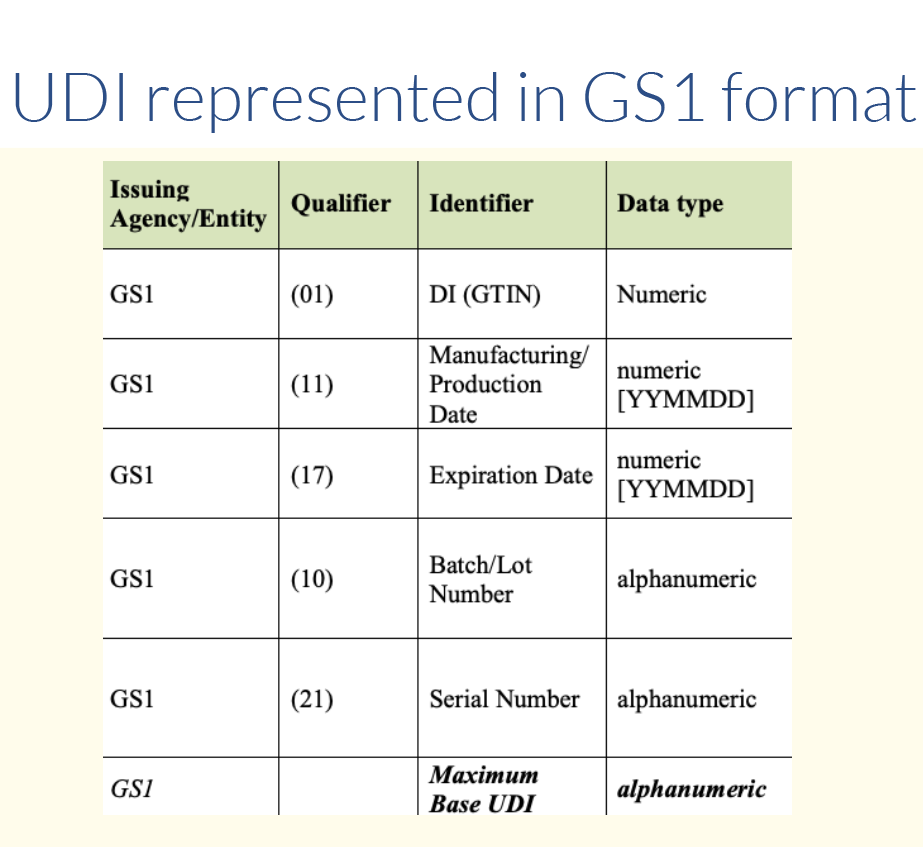
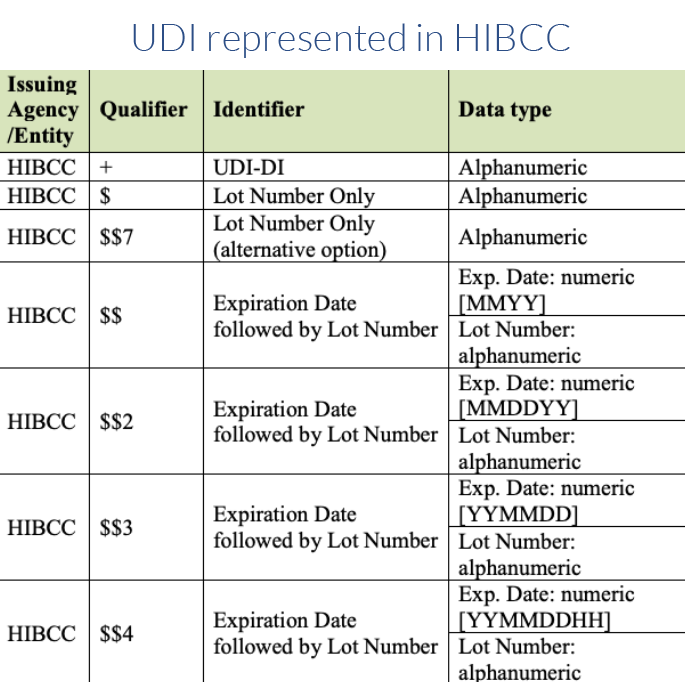
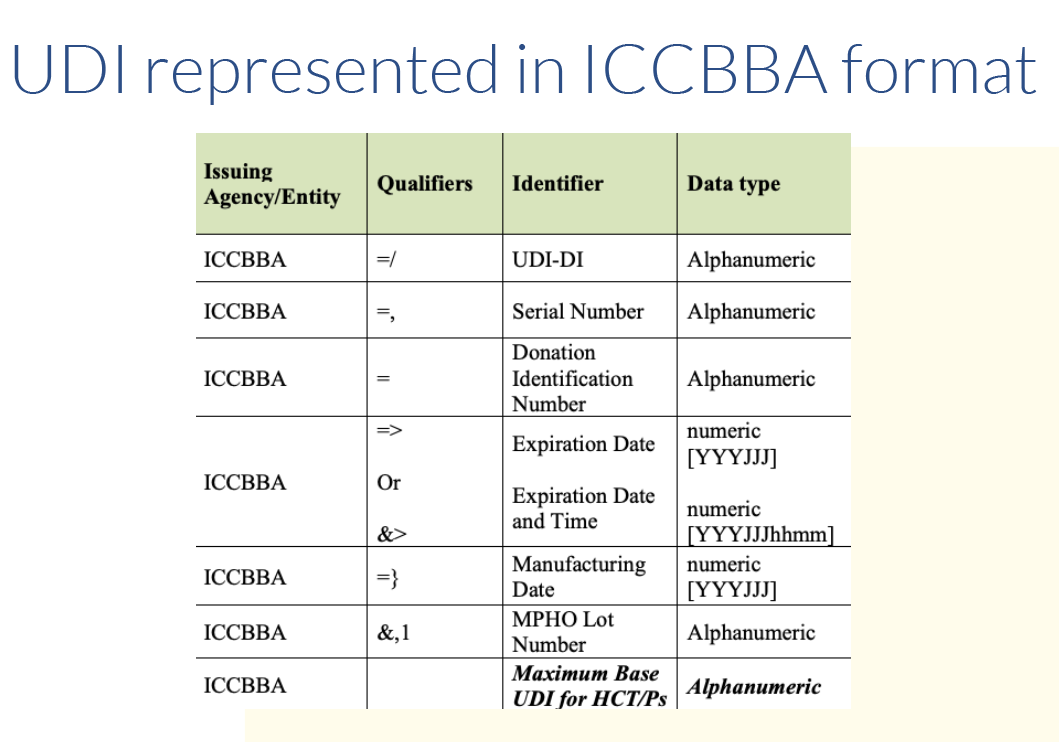
The product owner is responsible for obtaining the UDI from one of three approved issuing agencies. Those include GS1, HIBCC and ICCBBA for devices containing HCT/P. Each agency has its own format. Approximately 85% of the items in the Global UDI Database (GUDID) contain UDIs issued by GS1
 Click here to see Sarah Scott, Registered Nurse at Duke Raleigh Hospital explain which bar code to scan.
Click here to see Sarah Scott, Registered Nurse at Duke Raleigh Hospital explain which bar code to scan.
FDA and Issuing Agency Requirements:
The FDA requires all medical devices that do not have a specific exemption (e.g. contact lenses) to contain a UDI on their label, packages and in some cases on the product itself. The phase in period ended September 24, 2024.
The product owner is also responsible for populating specific data elements in the Global UDI Database (GUDID). This includes information on the existence of latex, MRI safety, the GMDN Preferred Term Name and other valuable information. The UDI-DI is the record key to link to this information. Please refer to the GUDID Data Elements Reference Table (DERT) for more information on data elements contained in the GUDID.
The FDA and the Issuing Agencies have rules that dictate when a UDI-DI must change. When changes occur the GUDID must be updated.
Each unit of measure must have its own UDI
